Courts take a fresh look at toxic priorities
The concept of multiple priorities, sometimes referred to as partial priority or split priority, has long been a matter for debate in European patent law (and thus UK patent law [see end note 1]). However, interest in how the law governing multiple priorities is to be interpreted has been stimulated in recent years due to the emergence of the toxic divisionals hypothesis and the toxic priority issue.
Over the last few years there have been a number of high profile decisions from both the Technical Boards of Appeal at the European Patent Office (EPO) and the UK courts, from which a divergent approach to the treatment of multiple priorities has emerged.
Accordingly, seventeen years on from G 2/98 – a seminal decision of the EPO's Enlarged Board of Appeal (EBoA) on the issue of priority – priority looks set to go before the EBoA again. In particular, Technical Board of Appeal 3.3.06 in case T 557/13 appears to have decided to refer one or more questions to the EBoA on the issue of multiple priorities. The interlocutory decision containing the referred questions is yet to be issued. However, as a prelude, this article provides a brief explanation of the legal principles behind the apportioning of multiple priorities and how the interpretation of these principles can affect the concepts of toxic priority and toxic divisionals.
Legal principles
Article 88(2) EPC provides that, "where appropriate, multiple priorities may be claimed for any one claim". However, no further guidance is provided in the implementing regulations as to when it is appropriate for a claim to be considered as having multiple priorities.
Decision G 2/98 (reason 6) provided guidance on when there may be justification for claiming multiple priorities for one and the same claim of an application. In particular, the EBoA drew a distinction between so called "AND"-claims and "OR"-claims.
With respect to "AND"-claims little difficultly has been encountered. Instead, much controversy concerning the apportioning of multiple priorities has focused on "OR-claims", ie, wherein claimed features are expressed in the alternative, in particular where alternative features are expressed by way of a generic feature.
Example 1
For example, apportioning of multiple priorities is straightforward in the situation as presented in example 1 below.
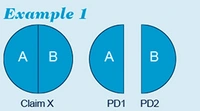
In example 1, claim X covering alternative embodiments A or B would be entitled to claim priority of application PD1 in respect of embodiment A and application PD2 in respect of embodiment B.
Example 2
However, the situation is less straightforward in the situation as depicted in example 2 below.
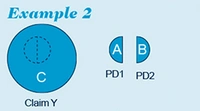
In example 2, claim Y generically covers alternative embodiments A or B. The question then arises to what extent claim Y is entitled to claim priority from application PD1 and/or application PD2, or whether it is entitled to the filing date only.
In this regard, the emphasis has been on Reason 6.7 of decision G 2/98, where it is stated that:
"The use of a generic term or formula in a claim for which multiple priorities are claimed in accordance with Article 88(2) EPC, second sentence, is perfectly acceptable under Articles 87(1) and 88(3) EPC, provided that it gives rise to the claiming of a limited number of clearly defined alternative subject-matters."
It is strict interpretation of the highlighted test which has led to the emergence of the toxic priority issue and the toxic divisional hypothesis.
Toxic priority/toxic divisionals
The toxic priority issue and toxic divisional hypothesis involve anticipation of at least one claim in an application/patent by the disclosure of its priority document or divisional/parent application respectively.
For anticipation to occur, the priority document or parent/divisional application must have an earlier effective date, must publish and must disclose subject matter falling within the scope of at least one claim in the reference application [see end note 2].
A scenario which could possibly give rise to a toxic priority issue is where the reference application claims an invention in broader terms than its published priority document (thus is not the 'same invention'). This may be the case when the applicant has been developing the invention during the priority year.
For example, the priority application (PD1) defines an invention by virtue of parameter Z having a value in the range 20 to 50. The invention is exemplified by a specific embodiment A wherein parameter Z is equal to 40.
The reference application is subsequently filed claiming priority of application PD1 where claim 1 is directed to the invention wherein the broader range for parameter Z of 10 to 50 is claimed.
If PD1 should publish, then claim 1 will be vulnerable to anticipation by embodiment A of PD1 if claim 1 of the reference application cannot claim multiple priorities, ie, be given the benefit of PD1 for the part range 20 to 50 and the filing date for 10 to 19, ie, the extension added at filing. In this case, embodiment A would not be prior art to claim 1. However, if it is not deemed appropriate to split the range 10 to 50 into sub-ranges for priority purposes, then claim 1 has a later effective date than embodiment A and thus embodiment A anticipates claim 1.
Regardless of whether PD1 publishes, the reference application may also be vulnerable with regard to its own divisional application. For instance, if a divisional application is filed with the same description as the parent reference application and thus contains embodiment A, when the divisional application publishes embodiment A disclosed therein becomes a disclosure which is potentially anticipatory to claim 1 of the parent for the same reasons as stated above.
Accordingly, the end result is dependent on whether the relevant authority consider it appropriate to split a claim into subject matter domains of varying priority date and how these priorities are apportioned.
Which brings us back to Reason 6.7 of G 2/98 and the interpretation of "provided that it gives rise to the claiming of a limited number of clearly defined alternative subject-matters."
Divergent approach
The majority of decided cases to date have concerned allegedly toxic priority documents. In only one decision to date (T 1496/11) has an application been held to be anticipated by the disclosure of its divisional application.
Furthermore, the majority of earlier decisions can be considered to follow a narrower interpretation of the test provided in Reason 6.7 of G 2/98.
The narrower interpretation of the test requires that the disclosure of the patent/application is analysed to determine if and how many priority domains the generic disclosure of the claim can be broken up into. This analysis is often guided by explicit disclosures of embodiments falling within the generic claims.
However, more recent decisions such as T 1222/11 and T 571/10, have seen the endorsement of a broader interpretation of the G 2/98 test. Based on the broader interpretation, determining whether an "OR"-claim can be awarded multiple priorities is independent of whether the subject matter or embodiment disclosed in the priority document is identified in the application as a separate embodiment. Rather, the broader interpretation proposes that it suffices for the purpose of claiming multiple priorities for it to be conceptually possible to identify the aforementioned limited number of clearly defined alternative subject matters.
Accordingly, we are left with the situation where under the narrower interpretation there is a danger of self collision due to toxic priority or toxic divisionals.
Alternatively, by adopting the broader interpretation, the incidence of toxic priority or toxic divisionals would appear less likely.
Consequently, due to the fundamental importance of multiple priorities and the potentially harsh consequences of applying them wrongly, the stage is set for the EBoA to resolve this important point of law.
Further guidance
Once the decision in T 557/13 is made available and the questions referred to the EBoA are known, we will provide a further update on this intriguing issue. In the meantime, should you have any questions please contact your usual D Young & Co advisor or the author or this article.
Notes
- Section 5 of the UK Patents Act 1977 (priority) is to be construed in conformity with the priority provisions of the EPC (Articles 87 to 89).
- In Europe and the UK, unpublished applications, including those by the applicant, in the same jurisdiction may be regarded as novelty only prior art if they have an earlier effective date and go on to publish. Certain jurisdictions (eg, US and Japan) avoid self collision by excluding the applicant's own unpublished application(s) from the contents of the state of the art.